Complete the world’s leading CT-FFR clinical research with Keya Medical’s independent artificial intelligence technology products: release of ACC.23 TARGET clinical research results.

In the early morning of March 5, Beijing time, the team of Professor Chen Yundai from the Cardiology Center of the General Hospital of the Chinese People’s Liberation Army published a special report on clinical research at the American Heart Association/World Cardiology (ACC/WCC)2023 Conference. Based on the artificial intelligence CT-FFR technology, the clinical research report on the treatment and follow-up of patients with stable coronary heart disease -TARGET trial, the results will be published simultaneously in the top international journals.CirculationJournal (TOP journal in JCR 1 area, impact factor 39.9).
The research was supported by the National Key R&D Program and the Beijing Science and Technology Rising Star Program, and combined with the research teams of cardiology and radiological imaging departments of several top domestic third-class first-class hospitals such as Beijing anzhen hospital affiliated to Capital Medical University, the Second Affiliated Hospital of Zhejiang University Medical College, Qilu Hospital of Shandong University, the First Affiliated Hospital of Xinjiang Medical University, and tongji hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology. The correspondent of this research paper is Professor Chen Yundai, Associate Professor Yang Junjie, Deputy Chief Physician Shan Dongkai and Dr. Wang Xi from the Department of Cardiovascular Medicine of PLA General Hospital are the co-first authors of this paper.
Introduction to research
TARGET study is the first multi-center, randomized and controlled clinical study in the world to evaluate the treatment and management of new stable chest pain patients using the field deployment strategy based on machine learning CT-FFR calculation. The research uses the artificial intelligence CT-FFR computing technology independently developed by China (Keya Medical Technology Co., Ltd.), and a total of 1216 patients from six medical centers in China were selected. The pretest probability of obstructive coronary heart disease in the enrolled patients was medium to high, and coronary CT angiography suggested that there was a critical stenosis of 30%-90%. The researchers randomly divided patients into CT-FFR diagnosis and treatment group (experimental group) or standard diagnosis and treatment group (control group). The main end point of the study was the proportion of patients with non-obstructive coronary artery disease or with obstructive coronary artery disease who did not receive revascularization during the follow-up coronary angiography within 90 days. Secondary end points included major adverse cardiovascular events, quality of life outcomes, improvement of angina symptoms and medical costs.
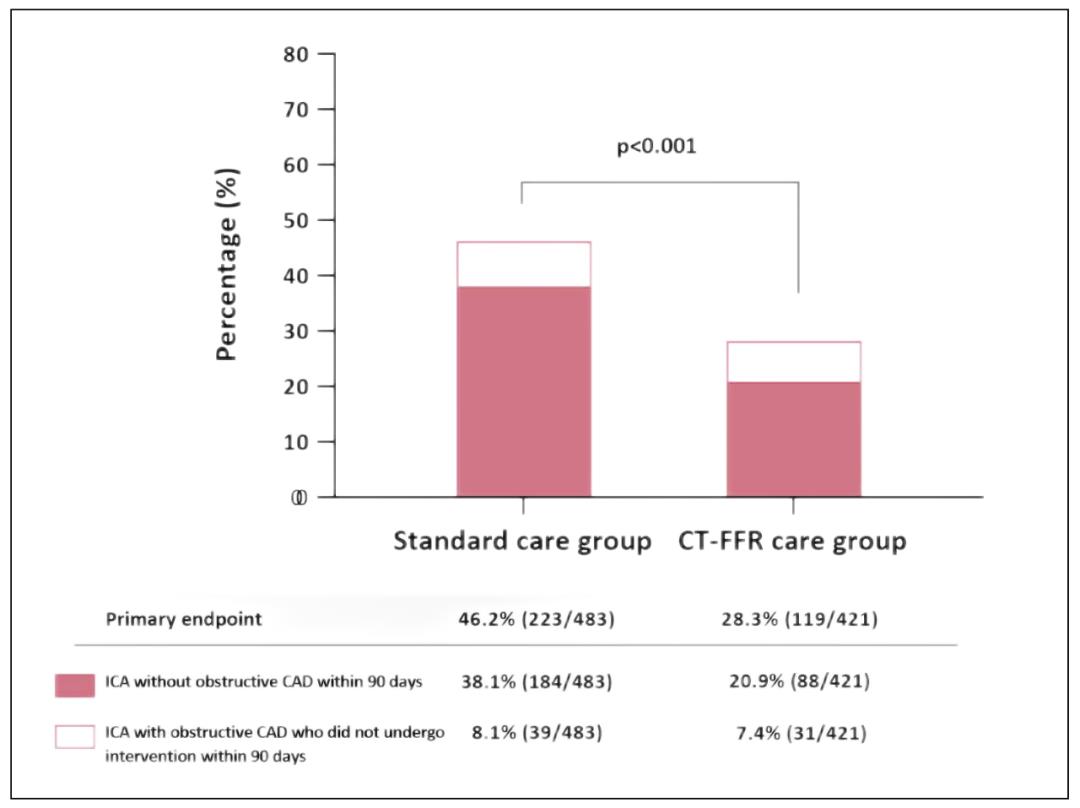
The results showed that compared with the control group, the proportion of patients with non-obstructive coronary disease or obstructive coronary disease who did not receive revascularization in CT-FFR diagnosis and treatment group decreased significantly (28.3% vs. 46.2%, P<0.001). On the whole, there were more patients receiving revascularization in CT-FFR group than in the control group (49.7% vs. 42.8%, P=0.02), but there was no significant difference in the proportion of MACE during the one-year follow-up (hazard ratio, 0.88; 95%CI, 0.59 to 1.30)。 During the follow-up period, the quality of life and symptoms of the two groups were similar, while the medical cost of CT-FFR treatment group tended to decrease. It is concluded that, compared with the standard diagnosis and treatment strategy represented by cardiac stress examination, the on-site deployment of CT-FFR calculation diagnosis and treatment strategy based on machine learning will significantly reduce the proportion of patients who have found non-obstructive coronary artery disease or do not need intervention within 90 days after coronary angiography. In addition, CT-FFR diagnosis and treatment strategy tends to save medical costs and increase the proportion of revascularization in the selected population. At the same time, CT-FFR strategy is consistent with the traditional path in improving patients’ symptoms or quality of life and the incidence of major clinical adverse vascular events.
Research enlightenment
TARGET research results show that "CT-FFR strategy based on machine learning in the field is feasible, safe and effective".
In the past 10 years, the extensive use of coronary CTA has promoted the diagnosis and treatment process of coronary heart disease in China. According to statistics, in 2017, the total number of coronary CTA angiography examinations in China reached 4.6 million. Therefore, the simple diagnostic function of coronary angiography is weakening, but among the patients who have received coronary angiography in China, most cases have not found obstructive coronary stenosis in the catheter room. Part of the reason for this phenomenon is that functional examination is not widely used or advanced cardiac imaging technology is not available enough. TARGET study further emphasizes that coronary angiography should only be applied to those patients who are most likely to have obstructive coronary stenosis or benefit from revascularization, and CT-FFR strategy will significantly optimize the management of stable coronary heart disease population.
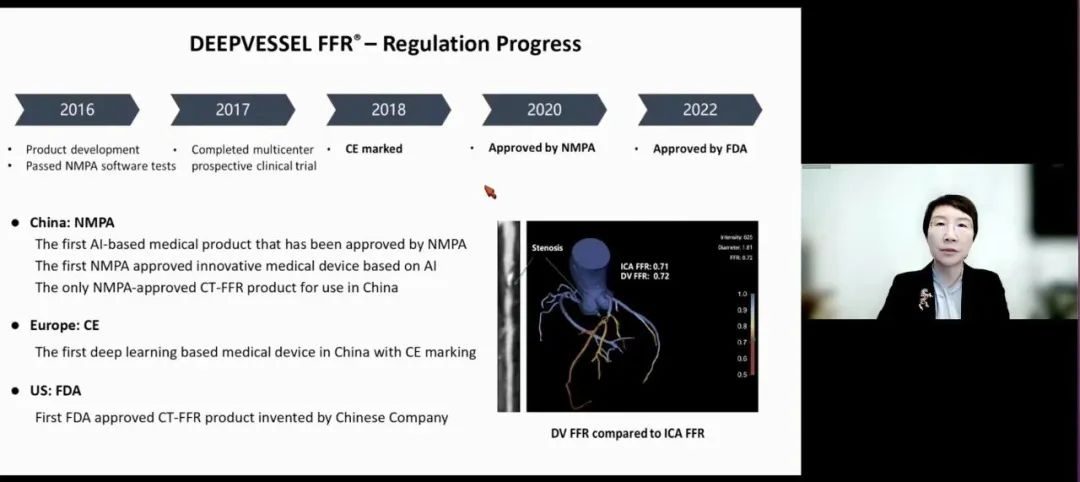
This study adopts the CT-FFR simulation calculation technology-deep pulse fraction independently developed by Keya Medical Technology Co., Ltd., uses deep learning technology to evaluate the physiological function of coronary artery, and uses artificial intelligence technology to evaluate the FFR of coronary angiography image, which is a deep learning technology independently developed and optimized based on the latest development in the field of computer vision. It can quickly and accurately analyze the non-invasive blood flow reserve fraction. In January 2020, this technology was approved as the first NMPA artificial intelligence medical device class III certificate in China, and now it has become the only CT-FFR product in the world that has been triple-certified by NMPA in China, CE in the European Union and FDA in the United States.
Pamela Douglas, MD (Duke Clinical Research Institute, Durham, North Carolina), former president of ACC, led the experiment funded by HeartFlow (PLATFORM and PRECISE research). She pointed out that the most outstanding thing about the TARGET experiment is the novelty of its on-site CT-FFR analysis.
It is indeed possible that the field deployment method is cheaper and can return the results faster. In clinical practice, if CCTA is used as a first-line test, Douglas said, then the question becomes: "If you have borderline lesions, what should you do next?" For her, "this is a little obvious, because CT-FFR is only a software analysis. Although the previous products are very complicated, it is not without risk to make an appointment for a load test and ask the patient to come back later."
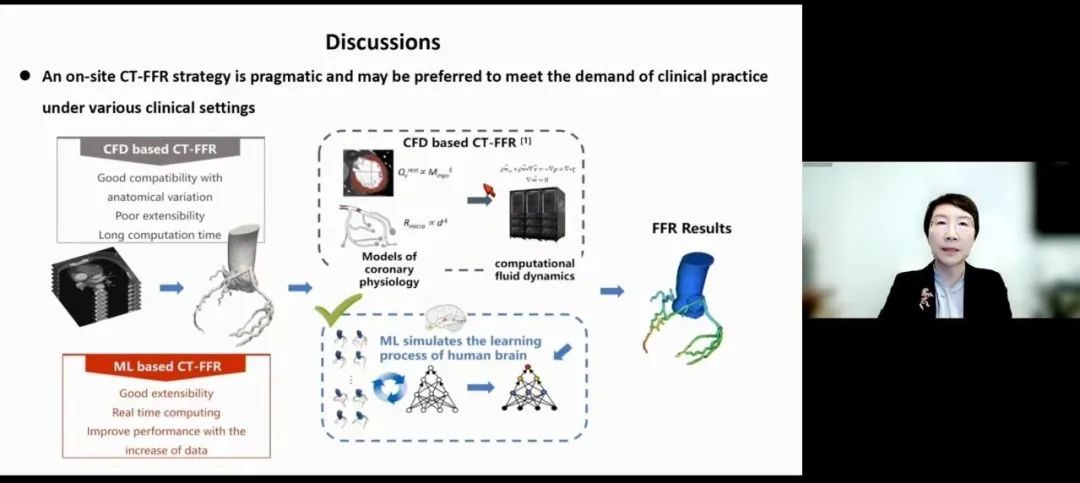
It is very important to deploy artificial intelligence computing in the field of TARGET research. "The advantage of using artificial intelligence algorithm is that it provides the possibility of field deployment, avoids the need to transfer sensitive medical data, shortens the calculation time and increases the participation of clinicians." They explained that although FFR can also be calculated by field computational fluid dynamics, this strategy is complex and requires a lot of resources. The convenience of machine learning will contribute to the application of CT-FFR in a wider range of scenarios, adding that "on-site CT-FFR strategy is practical and may be more suitable to meet the clinical practice needs in various clinical environments."